Cyclic polylactide synthesis initiated by a lithium anthraquinoid: understanding the selectivity through DFT and diffusion NMR†
Abstract
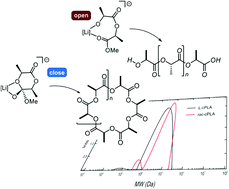
We report herein the first lithium anthraquinoid catalyst able to produce polylactide at room temperature starting from both L- and rac-lactide in the absence of any other initiator. The new lithium-based system is selective towards cyclic polymers, having narrow dispersity and weight-average molecular weights ranging from 49.5 to 137.6 kDa. The cyclic structure of the PLA generated from the lithium catalyst has been determined by a combination of 1H NMR and DRIFT spectroscopy techniques and MALDI-TOF mass spectrometry. DFT calculations point to lithium aggregation as the key feature of the catalyst controlling the linear : cyclic polymer ratio. Molar mass distributions and dispersities were obtained through diffusion NMR experiments combined with inverse Laplace transform algorithms and compared with those obtained by SEC as the most established method.


 Please wait while we load your content...
Please wait while we load your content...