Homo- and co-polymerisation of di(propylene glycol) methyl ether methacrylate – a new monomer†
Abstract
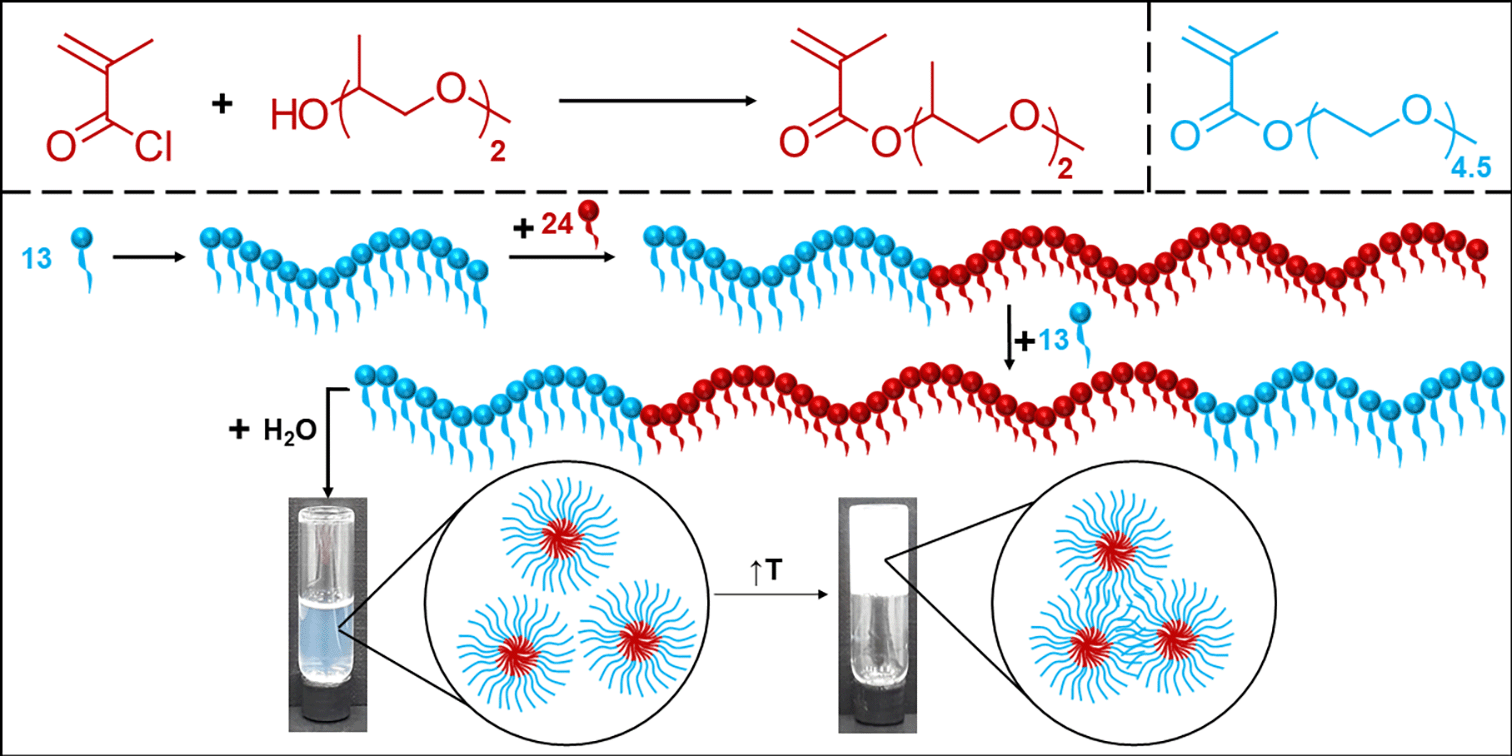
In this study, a new methacrylate monomer with two propylene glycol groups on the side chain, di(propylene glycol) methyl ether methacrylate (diPGMA), was synthesised via an esterification reaction. This new monomer was homo- and co-polymerised for the first time via group transfer polymerisation (GTP). Nine ABA triblock copolymers were synthesised via a “one-pot” GTP, with A and B blocks being based on the hydrophilic and the thermoresponsive oligo(ethylene glycol) methyl ether methacrylate, average Mn 300 g mol−1 (OEGMA300), and the hydrophobic diPGMA, respectively. The molar mass (MM) and OEGMA300/diPGMA content was systematically varied and the effect of this on the self-assembly and thermoresponsive properties was investigated. All copolymers were shown to self-assemble into aggregates and the size of these aggregates increased with both the MM of the polymer and the polymer content in diPGMA. A thermoresponse was observed in aqueous media, with the cloud point (CP) decreasing as the hydrophobic content increases, and the MM decreases. In concentrated aqueous solutions, the polymer with the highest MM and highest diPGMA content formed gels, whose storage modulus increases as a function of the concentration. This study reports a promising alternative hydrophobic monomer to be used in the fabrication of thermogelling materials.


 Please wait while we load your content...
Please wait while we load your content...