Poly(ionic liquid)s with superior swelling and enrichment properties in solvents†
Abstract
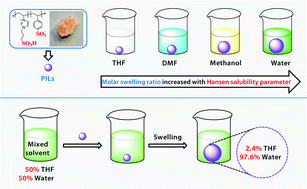
A series of poly(ionic liquid)s (PILs) based on 1-vinyl-3-(3-sulfopropyl)imidazolium (VSpIm) and sodium p-styrenesulfonate (NaSS) have been successfully synthesized and fully characterized. The swelling behaviours of the PILs in 18 solvents with a wide range of Hansen solubility parameters (δT) were investigated. The PILs exhibited distinct swelling properties in solvents with δT > 24.5 MPa1/2, such as water, formamide and methanol. The molar swelling ratio (QM), which represented the swelling ability of the PILs in solvent, was linearly positively correlated with the δT value. Moreover, the enrichment behaviours of the PILs in various mixed solvents were also studied. In water/solvent and formamide/solvent monophasic mixed solvents, the enrichment factors of the PILs to water and formamide were up to 39.82 (water/THF) and 17.69 (formamide/THF), respectively. The enrichment factor of the PILs to water (or formamide) was linearly positively correlated with the difference between δT (water) (or δT (formamide)) and δT (solvent). The excellent swelling and enrichment properties of the PILs in solvents were attributed to the strong interactions between the PILs and solvent molecules, especially the hydrogen-bonding interactions, which was supported by nuclear magnetic resonance (NMR) spectroscopy. Furthermore, a PIL was used as a catalyst for the hydration of alkyne and found that it had higher catalytic activity than those of both heterogeneous and homogeneous catalysts.


 Please wait while we load your content...
Please wait while we load your content...