Fluorinated nanotubes: synthesis and self-assembly of cyclic peptide–poly(vinylidene fluoride) conjugates†
Abstract
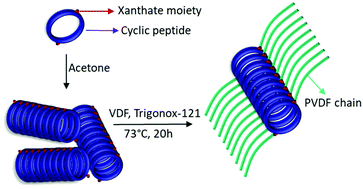
The synthesis of cyclic peptide–poly(vinylidene fluoride) (CP–PVDF) conjugates comprising (D-alt-L)-cyclopeptides as building blocks and their self-assembly into tube-like structures is described. By growing two PVDF polymeric chains from opposite sides of a preassembled cyclic-peptide macro-chain transfer agent, a PVDF–CP–PVDF conjugate was prepared. This “grafting-from” strategy, allowed the synthesis of the conjugate with high purity and using facile purification steps. The controlled self-assembly of the conjugate from DMF or DMSO solutions was carried out by addition of THF. This triggered the aggregation process that led to formation of tube-like structures. The mean length and width of the PVDF–CP–PVDF tubes were measured using atomic force microscopy (AFM) and transmission electron microscopy (TEM). Surprisingly, the self-assembly of the CP–PVDF conjugates in DMF/THF allowed the preparation of long (up to 25 μm) tube-like structures. The formation of such long tubular peptide–polymer aggregates, based on the stacking of cyclopeptides, is unprecedented and is believed to rely on synergetic effects between the stacking of the cyclic peptide and the interactions of the fluoropolymer–peptide conjugates.


 Please wait while we load your content...
Please wait while we load your content...