Introducing a 1,1-diphenylethylene analogue for vinylpyridine: anionic copolymerisation of 3-(1-phenylvinyl)pyridine (m-PyPE)†
Abstract
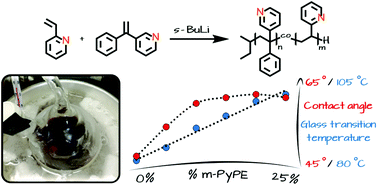
3-(1-Phenylvinyl)pyridine (m-PyPE), prepared by Wittig reaction from the readily available 3-benzoylpyridine, represents a structural analog of 1,1-diphenylethylene (DPE), one phenyl group being replaced by pyridine. The suitability of m-PyPE for the copolymerisation with vinylpyridine is reflected by the 13C NMR shifts of the β-carbon of 2-vinylpyridine (2-VP; 118.32 ppm) and m-PyPE (115.83 ppm, measured in CDCl3), which possess predictive character for carbanionic copolymerisation. In analogy to DPE and its manifold reported derivatives, carbanionic homopolymerisation of m-PyPE was not possible, due to its steric bulk. Copolymers of 2-VP and m-PyPE with varied composition have been synthesized with an incorporated amount of m-PyPE of up to 25%. Full conversion as well as the targeted incorporation ratio were verified by NMR experiments. Increasing the m-PyPE amount beyond 25% is possible, yet full monomer conversion and quantitative yields were not achieved anymore in this case. An increase of the glass transition temperatures of the resulting copolymers with increasing m-PyPE content was observed (DSC), and the difference in hydrophilicity compared to that of P(2-VP) was determined by contact angle measurements. m-PyPE offers potential also for functional termination of other living anionic polymerisations.


 Please wait while we load your content...
Please wait while we load your content...