Irgacure 2959-functionalized poly(ethyleneimine)s as improved photoinitiators: enhanced water solubility, migration stability and visible-light operation†
Abstract
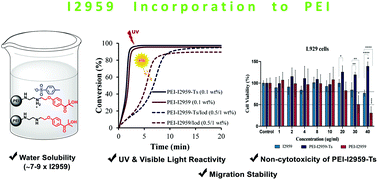
Two novel water soluble polymeric photoinitiators (PPIs) (PEI-I2959 and PEI-I2959-Ts) for free radical polymerization have been synthesized by introducing 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959 or I2959) functionality into branched poly(ethyleneimine) (Mw = 1800 g mol−1). These PPIs show an excellent water-solubility of 35–45 g L−1, thus exceeding the solubility of I2959 by 7–9-fold. PPIs show strong absorbance at 274 nm (ε ∼ 2–3 times larger than that of I2959); and less prominent peaks at 320 and 380 nm for PEI-I2959 and PEI-I2959-Ts, respectively, in water. The aza-Michael addition (∼10% conversion) and high photopolymerization efficiencies (90–100% conversion) under both UV and visible light are obtained by photo-differential scanning calorimetry (photo-DSC) and Real-Time Fourier Transform Infrared (RT-FTIR) spectroscopy. The photopolymerization mechanisms involve charge transfer complex formation, verified by absorption studies and enabling visible light polymerization; and a Type I mechanism. PEI-I2959-Ts exhibits 8 times higher migration stability than I2959 due to its high molecular weight. In tests against NIH/3T3 and L929 mouse embryonic fibroblast cells using the MTT cell viability assay, the non-cytotoxicity of PEI-I2959-Ts is shown and PEI-I2959 shows reasonable cell viability at lower concentrations, indicating that PPIs have potential usage in biomaterials.


 Please wait while we load your content...
Please wait while we load your content...