The effect of alkyl chain lengths on the red-to-near-infrared emission of boron-fused azomethine conjugated polymers and their film-state stimuli-responsivities†
Abstract
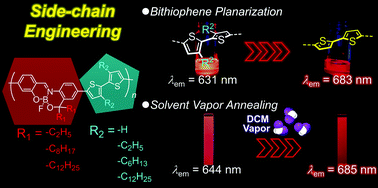
We present systematic studies of the dependencies of the red-to-near-infrared emission and stimuli-responsive properties of boron-fused azomethine (BAm) conjugated copolymers on the lengths of their alkyl side chains. We prepared a series of bithiophene copolymers with variable alkyl side chain lengths on the BAm and bithiophene moieties and evaluated their optical properties in solution and their stimuli-responsivity in films before and after vapor annealing. In the UV–vis absorption and emission spectra, it was shown that the elimination of the alkyl side chains on the bithiophene (BT) units led to a remarkable red-shift relative to that of the original polymer in the emission spectra. Theoretical calculations revealed that the enhancement of the planarity between the two thiophene rings played a significant role in the spectral red-shifting. Finally, a drastic red-shift in the emission (ca. 40 nm) was observed upon vapor annealing without a loss of emission efficiency (ΦF = 0.36).


 Please wait while we load your content...
Please wait while we load your content...