Purpurin derivatives as visible-light photosensitizers for 3D printing and valuable biological applications†
Abstract
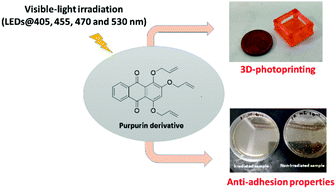
We report the synthesis of new visible-light photosensitizers derived from purpurin (mono-allyl- and triallyl-purpurin), and their use as type II photoinitiating systems when associated with N-methyldiethanolamine, bis(4-methylphenyl) iodonium hexafluorophosphate or tri-functionalized thiol (trimethylolpropane tris(3-mercaptopropionate)) for cationic and free-radical photopolymerization, and the initiation of thiol–ene reactions. These photoinitiating systems showed good initiating properties in laminate or under air upon visible-light exposure, i.e. LED@405 nm, 455 nm, 470 nm, 530 nm, and a Xe lamp. Steady-state photolysis, electron paramagnetic resonance, fluorescence analysis and laser flash photolysis have clearly highlighted the photochemical properties of the different photoinitiating systems and proved that purpurin derivatives could act as electron donors or as proton/proton-coupled electron transfer promoters when associated with appropriate additives. For the first time, we also demonstrated the capability of triallyl purpurin to produce newly designed 3D objects by 3D photoprinting technology. Interestingly, the photosensitizers from new 3D materials incorporating triallyl-purpurin have undoubtedly shown tremendous antibacterial properties with more than 99% of inhibition of bacterial adhesion upon visible-light exposure, even after many antibacterial cycling experiments, thus showing their capability to be recycled.


 Please wait while we load your content...
Please wait while we load your content...