Biobased polymers derived from itaconic acid bearing clickable groups with potent antibacterial activity and negligible hemolytic activity†
Abstract
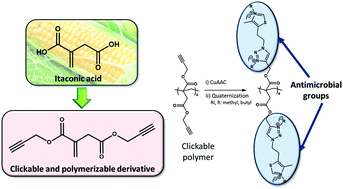
Herein, we report, for the first time, the synthesis of clickable polymers derived from biobased itaconic acid, which was then used for the preparation of novel cationic polymers with antibacterial properties and low hemotoxicity via click chemistry. Itaconic acid (IA) was subjected to chemical modification by incorporating clickable alkyne groups on the carboxylic acids. The resulting monomer with pendant alkyne groups was easily polymerized and copolymerized with dimethyl itaconate (DMI) by radical polymerization. The feed molar ratio of comonomers was varied to precisely tune the content of alkyne groups in the copolymers and the amphiphilic balance. Subsequently, an azide with a thiazole group, which is a component of the vitamin thiamine (B1), was attached onto the polymers by copper-catalyzed azide-alkyne cycloaddition (CuAAC) click chemistry leading to triazole linkages. N-Alkylation reactions of the thiazole and triazole groups with methyl and butyl iodides provide the corresponding itaconate derivatives with pendant azolium groups. The copolymers with variable cationic charge densities and hydrophobic/hydrophilic balances, depending on the comonomer feed ratio, display potent antibacterial activity against Gram-positive bacteria, whereas the activity was almost null against Gram-negative bacteria. Hemotoxicity assays demonstrated that the copolymers exhibited negligible hemolysis and excellent selectivity, more than 1000-fold, for Gram-positive bacteria over human red blood cells.


 Please wait while we load your content...
Please wait while we load your content...