New hole transport styrene polymers bearing highly π-extended conjugated side-chain moieties for high-performance solution-processable thermally activated delayed fluorescence OLEDs†
Abstract
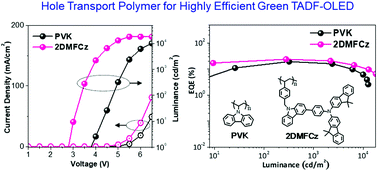
Two new hole transport styrene polymers, 2DMFCz and 2DBFCz, were successfully synthesized via radical polymerization. The design concept aims to investigate the hole-transporting ability and energy-level tunability by introducing bis(9,9-dimethyl-9H-fluoren-2-yl)aminocarbazole and bis(dibenzo[b,d]furan-2-yl)aminocarbazole as side-chain pendants. They were found to show good solubility in chlorobenzene but poor solubility in toluene, similar to poly(9-vinylcarbazole) (PVK). The highest occupied molecular orbital levels of 2DMFCz and 2DBFCz were determined to be −5.23 eV and −5.31 eV, respectively, while hole mobilities were estimated to be 1.65 × 10−7 cm2 V−1 s−1 and 1.48 × 10−8 cm2 V−1 s−1 measured by the space-charge limited-current method. Subsequently, in solution-processed green thermally activated delayed fluorescence organic light-emitting diodes (TADF-OLEDs), the 2DMFCz- and 2DBFCz-based devices exhibited a relatively low turn-on voltage of 2.7 V and higher maximum external quantum efficiencies of 23.84% and 21.11%, respectively. These values were superior to those of a PVK-based device. The polymer hole-transport materials presented in this study are promising materials that can play a significant role in improving the performance of TADF-OLEDs fabricated through a solution process in the future.


 Please wait while we load your content...
Please wait while we load your content...