Synthesis and self-assembly of photo-responsive polypeptoid-based copolymers containing azobenzene side chains†
Abstract
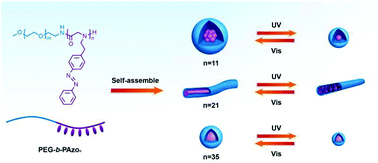
Stimuli-responsive polypeptoids are promising peptidomimetic polymers that can regulate their physical/chemical properties by means of external stimuli and have drawn tremendous attention for potential biotechnological applications. Here, we report the preparation of photo-responsive polypeptoids via the ring-opening polymerization of the monomer N-azobenzene-ethyl-N-carboxyanhydride (Azo-NNCA). The obtained diblock polypeptoids bearing azobenzene side chains (PEG-b-PAzo) showed reversible photo-responsive behavior in both organic and aqueous solutions, demonstrated by proton nuclear magnetic resonance (1H NMR) measurements and ultraviolet–visible (UV–vis) spectroscopy. Depending on the chain length of PAzo, various polymeric assemblies were readily obtained, including spherical and rod-like micelles, which exhibited a reversible morphology transformation upon alternating UV–vis light irradiation. The detailed process of morphology transformation was systemically investigated by transmission electron microscopy (TEM) and dynamic light scattering (DLS). Moreover, the polypeptoids also presented an irreversible responsiveness to temperature, resulting in different morphology transitions of assemblies. The new photo-responsive and biocompatible polypeptoid-based materials hold appreciable potential for biomedical and bioengineering applications.


 Please wait while we load your content...
Please wait while we load your content...