Transparent silphenylene elastomers from highly branched monomers†
Abstract
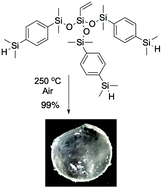
Thermally stable silicone elastomers based on silphenylenes are normally white solids at room temperature due to physical interactions between silphenylene moieties that lead to separated domains. We demonstrate that the Piers–Rubinsztajn reaction can be used to prepare highly branched tri- and tetrasilphenylene monomers from commercial alkoxysilanes and p-HSiMe2C6H4SiMe2H in the presence of B(C6F5)3. The H-terminated monomers could be cured to give soft elastomers simply by heating in air for up to 6 hours in the absence of a catalyst; curing occurs primarily via oxidative coupling of terminal SiH groups. The resulting elastomers were transparent (>95%) at room temperature up to 250 °C, and showed very good thermal stability (degradation begins >400 °C).


 Please wait while we load your content...
Please wait while we load your content...