Dithiocarbamation of spiro-aziridine oxindoles: a facile access to C3-functionalised 3-thiooxindoles as apoptosis inducing agents†
Abstract
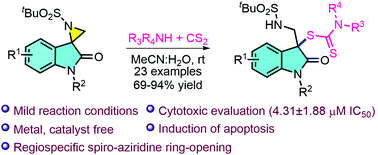
Herein, we report the first dithiocarbamation of spiro-aziridine oxindoles involving regiospecific ring-opening by using in situ generated nucleophilic dithiocarbamates as an instant source of sulfur. This approach afforded C3-functionalised-3-thiooxindoles in good to excellent yields with a wide substrate scope under catalyst-free and mild reaction conditions. These compounds were screened for their anticancer activity against a panel of human cancer cell lines, wherein compound 3u exhibited significant cytotoxic activity against human lung cancer cells with an IC50 value of 4.31 ± 1.88 μM. Phase contrast microscopy as well as different staining assays such as acridine orange/ethidium bromide (AO/EB), DAPI and DCFDA demonstrated the induction of apoptosis in A549 lung cancer cells after treatment with compound 3u. In addition, the clonogenic assay and migration assay demonstrated the ability of compound 3u to inhibit colony formation and cell migration, respectively, in A549 cells in a dose-dependent manner.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...