Amide bond formation in aqueous solution: direct coupling of metal carboxylate salts with ammonium salts at room temperature†
Abstract
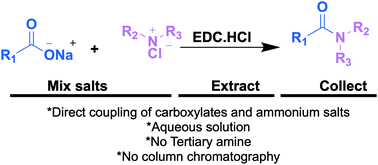
Herein, we report a green, expeditious, and practically simple protocol for direct coupling of carboxylate salts and ammonium salts under ACN/H2O conditions at room temperature without the addition of tertiary amine bases. The water-soluble coupling reagent EDC·HCl is a key component in the reaction. The reaction runs smoothly with unsubstituted/substituted ammonium salts and provides a clean product without column chromatography. Our reaction tolerates both carboxylate (which are unstable in other forms) and amine salts (which are unstable/volatile when present in free form). We believe that the reported method could be used as an alternative and suitable method at the laboratory and industrial scales.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...