Mechanistic insights into lysine-targeting covalent inhibition through a theoretical study of ester aminolysis†
Abstract
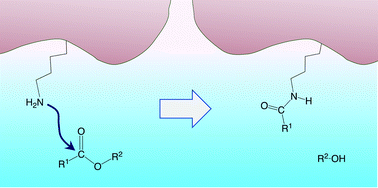
Development of targeted covalent inhibitors in drug design has a broad and important interest and many efforts are currently being made in this direction. Targeted covalent inhibitors have special relevance in oncology due to the possibilities they offer to overcome the problems of acquired resistance. In recent experiments, lysine-targeting has been envisaged for the irreversible inhibition of the heterodimeric lipid kinase phosphoinositide 3-kinase delta (PI3Kδ). Activated esters have been evaluated and shown to be promising inhibitors of this enzyme, but the reaction mechanisms display specificities that are not yet fully understood. In the present work, we have carried out a theoretical study of the aminolysis reaction of model esters in aqueous solution to gain insights into the corresponding biological processes. We have found that phenolic esters bearing electron-withdrawing groups are particularly reactive. The predicted mechanism involves the formation of a tetrahedral zwitterionic intermediate, which dissociates into an alkoxide and a protonated amide, this charge separation being the driving force for the subsequent proton transfer and final product formation. Structure–reactivity relationships are reported and shown to be a useful tool for evaluating potential inhibitor candidates.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...