Stereocomplementary synthesis of casuarine and its 6-epi-, 7-epi-, and 6,7-diepi-stereoisomers†
Abstract
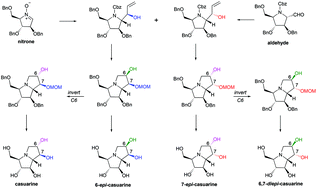
Four diastereomers belonging to the family of casuarines, including casuarine (1), 6-epi-casuarine (2), 7-epi-casuarine (13) and 6,7-diepi-casuarine (14), have been synthesized from D-arabinose-derived cyclic nitrone 7 and nitrone-derived aldehyde 4 by a stereocomplementary strategy. Glycosidase inhibition comparison showed that 6-epi-casuarine (2) exhibits enhanced inhibition of trehalase (IC50 = 9.7 μM) and 6,7-diepi-casuarine (14) leads to specific inhibition of trehalase.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...