Carbene functionalization of porphyrinoids through tosylhydrazones†
Abstract
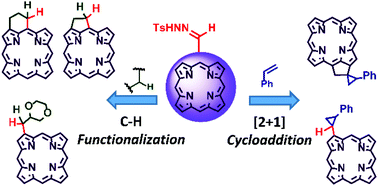
Here, we investigated methods for carbene functionalization of porphyrinoids through metal catalyst-free thermal decomposition of their tosylhydrazones. For the first time, tetrapyrrolyl substituted carbenes were obtained via thermolysis of tosylhydrazones of the corresponding tetrapyrrolyl aldehydes and ketones in the presence of a base. The carbenes formed reacted thermally with substrates without a metal catalyst or light irradiation. Carbenes at the β-pyrrolic position of porphyrinoids reacted with styrene leading to cyclopropane derivatives of tetrapyrroles. Carbenes also reacted with 1,4-dioxane with their insertion into the C–H bond yielding a tetrapyrrole 1,4-dioxane conjugate. Thermolysis of tosylhydrazones of meso-formyl-β-octaalkylporphyrinoids led exclusively to the corresponding cyclopentane fused porphyrinoids via intramolecular carbene C–H insertion. A plausible reaction mechanism was discussed based on DFT calculations of the intermediates. The tetrapyrrolyl carbenes were found to be considerably more stable than other carbenes. The products of the functionalization of porphyrinoids via hydrazone formation and subsequent carbene reactions exhibited modified optical spectra. The method for carbene functionalization of porphyrinoids through thermal decomposition of their tosylhydrazones created a new synthetic pathway for tailoring the perimeter of tetrapyrrolic macrocycles. Moreover, this method allows the obtainment of dyes with controllable spectral optical properties. In particular, new tetrapyrrole derivatives possessing phytoporphyrin carbon skeletons which have not been accessible were obtained using a convenient straightforward procedure.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...