Synthesis of chiral branched allylamines through dual photoredox/nickel catalysis†
Abstract
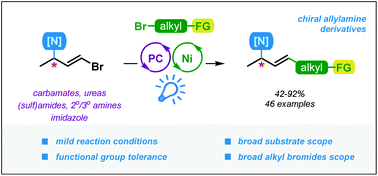
Allylamines are versatile building blocks in the synthesis of various naturally occurring products and pharmaceuticals. In contrast to terminal allylamines, the methods of synthesis of their branched congeners with internal, stereodefined double bonds are less explored. This work describes a new approach for the preparation of allylamines via cross-coupling of alkyl bromides with simple 3-bromoallylamines by merging the photoredox approach and Ni catalysis. The reaction proceeds under mild conditions, under blue light irradiation, and in the presence of an organic dye, 4CzIPN, as a photocatalyst. The scope of suitable reaction partners is broad, including alkyl bromides bearing reactive functionalities (e.g., esters, nitriles, aldehydes, ketones, epoxides) and N-protected allylamines, as well as N-allylated secondary and tertiary amines and heterocycles. The employment of non-racemic starting materials allows for rapid and easy construction of complex multifunctional allylamine derivatives without the loss of enantiomeric purity.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...