Effective synthesis of novel dihydrobenzisoxazoles bearing the 2-aminothiazole moiety and evaluation of the antiproliferative activity of their acylated derivatives†
Abstract
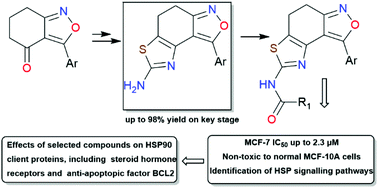
An effective method for the synthesis of 8-aryl-4,5-dihydrothiazolo[4′,5′:3,4]benzo[1,2-c]isoxazol-2-amines was developed. This method includes the α-keto bromination of 3-aryl-6,7-dihydrobenzo[c]isoxazol-4(5H)-ones followed by the condensation of the obtained bromo derivatives with thiourea in acetonitrile. Using virtual screening, a series of acylated derivatives of the obtained compounds were selected as potential HSP90 inhibitors. These compounds were prepared and evaluated as antiproliferative agents against three cancer cell lines (A431, 22Rv1, and MCF-7). Compounds 8b, 8c and 8q exhibiting high antiproliferative potency against MCF-7 breast cancer cells with IC50 values ranging from 2.3 to 9.5 μM were chosen for in-depth evaluation. The selected compounds had remarkable effects on HSP90 client proteins, including steroid hormone receptors and the anti-apoptotic factor BCL2. The obtained compounds are of interest for anticancer drug development.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...