A glutamic acid-based traceless linker to address challenging chemical protein syntheses†
Abstract
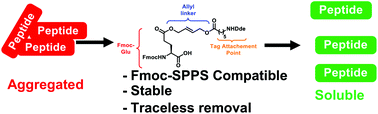
Native chemical ligation (NCL) enables the total chemical synthesis of proteins. However, poor peptide segment solubility remains a frequently encountered challenge. Here we introduce a traceless linker that can be temporarily attached to Glu side chains to overcome this problem. This strategy employs a new tool, Fmoc-Glu(AlHx)-OH, which can be directly installed using standard Fmoc-based solid-phase peptide synthesis. The incorporated residue, Glu(AlHx), is stable to a wide range of chemical protein synthesis conditions and is removed through palladium-catalyzed transfer under aqueous conditions. General handling characteristics, such as efficient incorporation, stability and rapid removal were demonstrated through a model peptide modified with Glu(AlHx) and a Lys6 solubilizing tag. Glu(AlHx) was incorporated into a highly insoluble peptide segment during the total synthesis of the bacteriocin AS-48. This challenging peptide was successfully synthesized and folded, and it has comparable antimicrobial activity to the native AS-48. We anticipate widespread use of this easy-to-use, robust linker for the preparation of challenging synthetic peptides and proteins.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...