Disulfide metathesis via sulfur⋯iodine interaction and photoswitchability†
Abstract
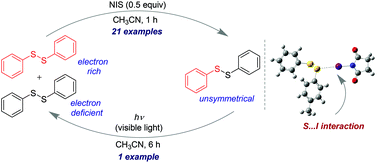
The idea of constitutional dynamic chemistry (CDC) and dynamic combinatorial chemistry (DCC) is widespread in the literature using the chemistry of disulfides. The synthesis of unsymmetrical diaryl disulfides is challenging due to the presence of a weak S–S bond. We report herein the synthesis of unsymmetrical diaryl disulfides from two symmetrical disulfides via a cross-metathesis reaction which was controlled by a weak sulfur⋯iodine (S⋯I) interaction. The unsymmetrical disulfides were stable in acetonitrile solution in the presence of N-iodosuccinimide (NIS), and found to be reversibly photoswitchable to the symmetrical disulfides under visible light irradiation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...