Photoredox-catalyzed redox-neutral difluoroalkylation to construct perfluoroketones with difluoroenoxysilanes†
Abstract
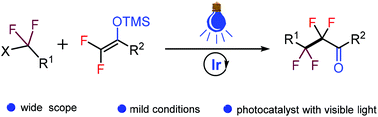
A mild and facile approach to construct various perfluoroketones via photo-catalyzed difluoroalkylation of difluoroenoxysilanes is developed. The reaction includes a strategy of combination of two fluorine-containing functional groups, which confers the reaction with characteristics like high efficiency, mild conditions, and broad scope. A variety of fluoroalkyl halides including perfluoroalkyl iodides, bromo difluoro esters and amides can be employed as radical precursors. Control experiments indicate that a single-electron transfer pathway may be involved in the reaction.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...