Highly diastereoselective entry to chiral oxindole-based β-amino boronic acids and spiro derivatives†
Abstract
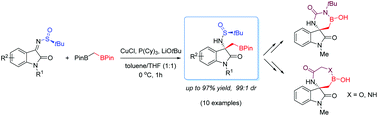
We here describe the first Cu-catalysed, diastereoselective 1,2-addition of 1,1-diborylmethane to chiral ketimines for the synthesis of quaternary stereocenters and spiro compounds. The method provides easy access to a range of chiral, highly functionalized compounds, namely oxindole-based β,β′-disubstituted β-amino boronates, boron-containing peptidomimetics and six-, seven-membered spirocyclic hemiboronic esters. Such unprecedented compounds are mostly obtained in high yields and easily isolated as single diastereoisomers, paving the way to a more intense exploitation of boron-containing compounds in diversity-oriented chemistry and drug-discovery programs. Concerning stereochemistry, the application of Ellman's auxiliary strategy allows in principle to access both steric series of target compounds.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...