Rhodium(iii)-catalyzed synthesis of trisubstituted furans via vinylic C–H bond activation†
Abstract
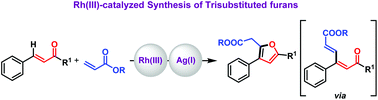
We report an Rh(III)-catalyzed one-pot synthesis of trisubstituted furan derivatives through Cvinyl–H activation of α,β-unsaturated ketones with acrylates. The control study revealed that the Heck-type product obtained undergoes Paal–Knorr type cyclization in the presence of an Ag salt. Hence, the Ag salt plays a dual role of a halide scavenger and a Lewis acid catalyst for Paal–Knorr type cyclization. The furan product can be transferred into the respective alcohol and acid derivatives which are useful intermediates in synthesizing biologically active molecules.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...