Tautomers of N-acetyl-d-allosamine: an NMR and computational chemistry study
Abstract
D-Allosamine is a rare sugar in Nature but its pyranoid form has been found α-linked in the core region of the lipopolysaccharide from the Gram-negative bacterium Porphyromonas gingivalis and in the chitanase inhibitor allosamidin, then β-linked and N-acetylated. In water solution the monosaccharide N-acetyl-D-allosamine (D-AllNAc) shows a significant presence of four tautomers arising from pyranoid and furanoid ring forms and anomeric configurations. The furanoid ring forms both showed 3JH1,H2 ≈ 4.85 Hz and to differentiate the anomeric configurations a series of chemical shift anisotropy/dipole–dipole cross-correlated relaxation NMR experiments was performed in which the α-anomeric form showed notable different relaxation rates for its components of the H1 doublet, thereby making it possible to elucidate the anomeric configuration of each of the furanoses. The conformational preferences of the different forms of D-AllNAc were investigated by 3JHH, 2JCH and 3JCH coupling constants from NMR experiments, molecular dynamics simulations and density functional theory calculations. The pyranose form resides in the 4C1 conformation and the furanose ring form has the majority of its conformers located on the South–East region of the pseudorotation wheel, with a small population in the Northern hemisphere. The tautomeric equilibrium was quite sensitive to changes in temperature, where the β-anomer of the pyranoid ring form decreased upon a temperature increase while the other forms increased.
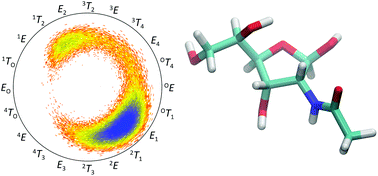
- This article is part of the themed collections: Chemical Biology in OBC and Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...