Microfluidic synthesis of pyrrolidin-2-ones via photoinduced organocatalyzed cyclization of styrene, α-bromoalkyl esters and primary amines†
Abstract
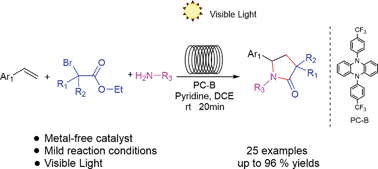
A green and efficient reaction route for the synthesis of pyrrolidin-2-ones via photoinduced organocatalyzed three-component cyclization of styrene, tertiary α-bromoalkyl esters and primary amines in a microchannel reactor under visible light conditions has been developed. Moreover, the overall process can be carried out under mild conditions without using a metal. In addition, a reasonable reaction mechanism was proposed based on control experiments.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...