Copper-promoted direct amidation of isoindolinone scaffolds by sodium persulfate†
Abstract
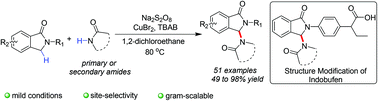
Isoindolinones are ubiquitous structural motifs in natural products and pharmaceuticals. Establishing an efficient method for structural modification of isoindolinones could significantly facilitate new drug development. Herein, we describe copper-promoted direct amidation of isoindolinone scaffolds mediated by sodium persulfate. The method exhibits mild reaction conditions and high site-selectivity, and enables the structural modification of the drug indobufen ester with various amides with yields of 49 to 98%. It is also gram-scalable. Additionally, the reaction mechanism appears to involve a radical and a carbocationic pathway.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...