Trifluoroethanol-promoted ring-opening cyclization of 4-(2-oxiranylmethoxy)indoles: access to 4,5-fused indoles†
Abstract
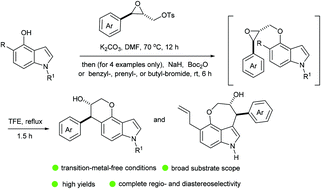
Herein, we report that the trifluoroethanol-mediated ring-opening cyclization of readily accessible 4-(2-oxiranylmethoxy)indoles takes place in a diastereoselective and 6-endo fashion to generate pyrano[2,3-e]indol-3-ols in high yields. This regioselective cyclization at the indole C-5 position requires the presence of a π-activating aryl substituent on the reacting epoxide carbon atom, but remains uninfluenced by the electronic nature of the indole-N-substituent. Interestingly, blocking the C-5 position of the indole unit directs the reaction to generate oxepino[4,3,2-cd]indol-3-ols via 7-endo epoxide–arene cyclization.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...