One-pot synthesis of N-substituted benzannulated triazoles via stable arene diazonium salts†
Abstract
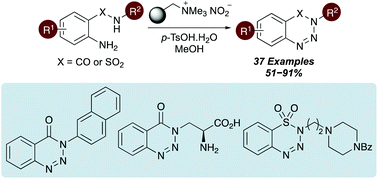
A mild and effective one-pot synthesis of 1,2,3-benzotriazin-4(3H)-ones and benzothiatriazine-1,1(2H)-dioxide analogues has been developed. The method involves the diazotisation and subsequent cyclisation of 2-aminobenzamides and 2-aminobenzenesulfonamides via stable diazonium salts, prepared using a polymer-supported nitrite reagent and p-tosic acid. The transformation was compatible with a wide range of aryl functional groups and amide/sulfonamide-substituents and was used for the synthesis of pharmaceutically important targets. The synthetic utility of the one-pot diazotisaton–cyclisation process was further demonstrated with the preparation of an α-amino acid containing 1,2,3-benzotriazin-4(3H)-one.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...