Metal-free transamidation of benzoylpyrrolidin-2-one and amines under aqueous conditions†
Abstract
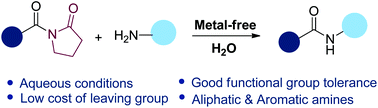
N-Acyl lactam amides, such as benzoylpyrrolidin-2-one, benzoylpiperidin-2-one, and benzoylazepan-2-one reacted with amines in the presence of DTBP and TBAI to afford the transamidated products in good yields. The reactions were conducted under aqueous conditions and good functional group tolerance was achieved. Both aliphatic and aromatic primary amines displayed good activity under metal-free conditions. A radical reaction pathway is proposed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...