Iridium-catalyzed regioselective hydrosilylation of internal alkynes facilitated by directing and steric effects†
Abstract
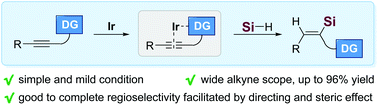
Here we reported the iridium-catalyzed hydrosilylation of internal alkynes under simple and mild conditions. The intrinsic functional groups of alkyne substrates were disclosed to be crucial in facilitating both the hydrosilylation process and related regioselectivity owing to their coordination capability towards the iridium catalyst. Utilization of the steric trimethylsilyl-protected trihydroxysilane proved to be another critical factor in ensuring the efficient proceeding of this process.


 Please wait while we load your content...
Please wait while we load your content...