Syntheses of 4-allyl-/4-allenyl-4-(arylthio)-1,4-dihydroisoquinolin-3-ones via the photochemical Doyle–Kirmse reaction†
Abstract
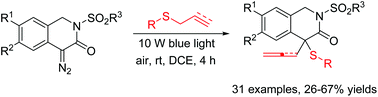
Facile synthesis of 4-allyl-/4-allenyl-4-(arylthio)-1,4-dihydroisoquinolin-3-ones via the visible-light-induced Doyle–Kirmse reaction of 4-diazo-1,4-dihydroisoquinolin-3-ones with allyl-/propargyl sulfides is reported. The reaction proceeds via the generation of free carbenes from cyclic diazo compounds followed by in situ formation of sulfonium ylide intermediates, which subsequently undergo [2,3-sigmatropic rearrangement] to give highly functionalized dihydroisoquinolinones in moderate to good yields. Broad substrate scope, and catalyst-free and mild conditions are the merits of this reaction.


 Please wait while we load your content...
Please wait while we load your content...