1,10/1,11-Cyclization catalyzed by diverged plant sesquiterpene synthases is dependent on a single residue†
Abstract
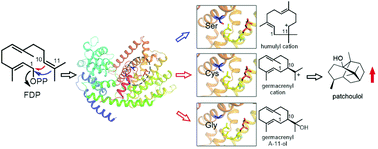
The exquisite chemodiversity of terpenoids is the product of the large diverse terpene synthase (TPS) superfamily. Here, by using structural and phylogenetic analyses and site-directed mutagenesis, we identified a residue (Cys440 in Nicotiana tabacum 5-epi-aristolochene synthase) proximal to an ion-binding motif common to all TPSs and named the preNSE/DTE residue, which determines the product specificity of sesquiterpene synthases from different plant species. In sesquiterpene synthases catalyzing 1,10-cyclization (1,10-cyclases) of farnesyl diphosphate, mutation of the residue in both specific and promiscuous 1,10-cyclases from different lineages leads to the accumulation of monocyclic germacrene A-11-ol, which is “short-circuited” from complex cyclization cascades, suggesting a key role of this residue in generating the first common intermediate of 1,10-cyclization. Altering this residue in a specific 1,11-cyclase results in alternative 1,10-cyclization products. Moreover, the preNSE/DTE residue can be harnessed to engineer highly specific sesquiterpene synthases for an improved proportion of high-value terpenoids, such as patchoulol, a main constituent of several traditional Chinese medicines that could treat SARS-CoV-2.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...