Sequential condensation/biannulation reactions of β-(2-aminophenyl)-α,β-ynones with 1,3-dicarbonyls†
Abstract
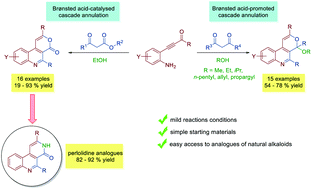
A divergent domino condensation/biannulation reaction of β-(2-aminophenyl) α,β-ynones with 1,3-dicarbonyls to construct a polycyclic 4H-pyrano[3,4-c]quinoline core has been developed. The p-TsOH·H2O catalyzed reaction of β-(2-aminophenyl) α,β-ynones with β-ketoesters in ethanol proceeds with good to excellent yields to provide a simple and effective method for the synthesis of functionalized 4H-pyrano[3,4-c]quinolinones. Further elaboration of these latter derivatives with an excess of 20% NH4OH in EtOH at 50 °C helps achieve the synthesis of the perlodinine analogues benzo[c][2,7]naphthyridin-4(3H)-one derivatives in high yields. Moreover, the p-TsOH·H2O mediated reaction of β-(2-aminophenyl) α,β-ynones with β-di-ketones leads to the formation of a variety of structurally diverse 4H-pyrano[3,4-c]quinoline polycyclic ketals by the incorporation of an alcohol solvent molecule in a cascade fashion.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...