Synthesis of a biotinylated heptose 1,7-bisphosphate analogue, a probe to study immunity and inflammation†
Abstract
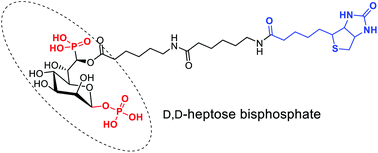
D-glycero-D-manno-Heptose-1β,7-bisphosphate (HBP) is a bacterial metabolite that can induce a TIFA-dependent innate immune response in mammals. It was recently discovered that after HBP enters into the cytoplasm of the host cell, it is transformed into ADP-heptose-7-phosphate, which then leads to ALPK1-TIFA-dependent inflammatory response. In order to provide a molecular tool allowing the discovery of the proteins involved in this novel inflammatory pathway, we designed and synthesized a biotinylated analogue of HBP. This chemical probe displays an anomeric β-phosphate and a phosphonate at the 7-position, and a D-configured 6-position to which is attached the biotin moiety. To do so, different synthetic strategies were explored and described in this report. Moreover, we demonstrated that the biotinylated version of HBP is still biologically active and can activate the NF-κB pathway in HEK293T cells.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...