Facile synthesis of indolizinoindolone, indolylepoxypyrrolooxazole, indolylpyrrolooxazolone and isoindolopyrazinoindolone heterocycles from indole and imide derivatives†
Abstract
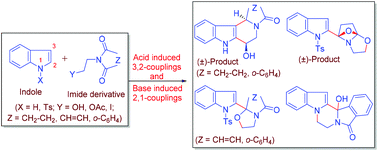
Chemo-, regio- and diastereoselective coupling reactions of indole with imide derivatives leading to unique heterocyclic systems are demonstrated. Acid-induced 3-position coupling reactions of indole with cyclic imide derived lactamols followed by acid promoted 2-position cyclizations with the corresponding aldehydes are described to obtain the indolizinoindolones and benzoindolizinoindolones. Base induced 2-position coupling reactions of N-tosylindole with N-(2-iodoethyl)imides and the subsequent cyclizations provide indolylepoxypyrrolooxazole, indolylpyrrolooxazolone and indolyloxazoloisoindolone. Reductive cleavage of indolyloxazoloisoindolone to the corresponding alcohol followed by mesylation and base promoted N-cyclization affords the in situ air-oxidized pentacyclic product hydroxyisoindolopyrazinoindolone. A regioisomeric structural revision of the natural product from 1,2,5,6,7,11c-hexahydro-3H-indolizino[7,8-b]indol-3-one to 1,2,5,6,11,11b-hexahydro-3H-indolizino(8,7-b)indol-3-one is also reported in the present studies focussed on the methodologies for heterocyclic synthesis.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...