CF3SO3H-enabled cascade ring-opening/dearomatization of indole derivatives to polycyclic heterocycles†
Abstract
A novel dearomatization process to produce fused polycyclic indolines via a CF3SO3H-mediated cascade ring-opening of a β-lactam and hydroaminative cyclization is demonstrated. It provides a new strategy for the synthesis of important polycyclic indoline-2-amine derivatives in moderate to excellent yields, as well as with good functional group tolerance. Moreover, transformation of the product was performed to deliver the corresponding acid, alcohol and amide smoothly.
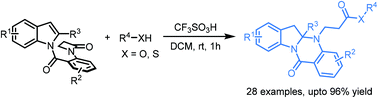
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...