Synthesis, biological evaluation and molecular modeling of urea-containing MraY inhibitors†
Abstract
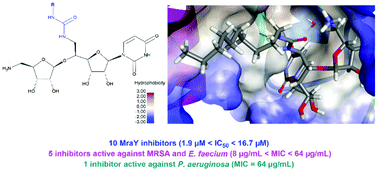
The straightforward synthesis of aminoribosyl uridines substituted by a 5′-methylene-urea is described. Their convergent synthesis involves the urea formation from various activated amides and an azidoribosyl uridine substituted at the 5′ position by an aminomethyl group. This common intermediate resulted from the diastereoselective glycosylation of a phthalimido uridine derivative with a ribosyl fluoride as a ribosyl donor. The inhibition of the MraY transferase activity by the synthetized 11 urea-containing inhibitors was evaluated and 10 compounds revealed MraY inhibition with IC50 ranging from 1.9 μM to 16.7 μM. Their antibacterial activity was also evaluated on a panel of Gram-positive and Gram-negative bacteria. Four compounds exhibited a good activity against Gram-positive bacterial pathogens with MIC ranging from 8 to 32 μg mL−1, including methicillin resistant Staphylococcus aureus (MRSA) and Enterococcus faecium. Interestingly, one compound also revealed antibacterial activity against Pseudomonas aeruginosa with MIC equal to 64 μg mL−1. Docking experiments predicted two modes of positioning of the active compounds urea chain in different hydrophobic areas (HS2 and HS4) within the MraY active site from Aquifex aeolicus. However, molecular dynamics simulations showed that the urea chain adopts a binding mode similar to that observed in 5CKR structural model and targets the hydrophobic area HS2.


 Please wait while we load your content...
Please wait while we load your content...