Acid-catalyzed oxidative cross-coupling of acridans with silyl diazoenolates and a Rh-catalyzed rearrangement: two-step synthesis of γ-(9-acridanylidene)-β-keto esters†
Abstract
A MsOH-catalyzed oxidative cross-coupling of acridans and silyl diazoenolates and a Rh2(OAc)4-catalyzed rearrangement of the resultant diazo products are described. The reactions provide various γ-(9-acridanylidene)-β-keto esters in good yields, which bear an active α-methylene unit for further functionalization.
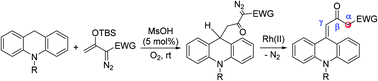
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...