Diversity-orientated synthesis of macrocyclic heterocycles using a double SNAr approach†
Abstract
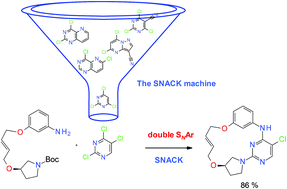
An efficient macrocyclisation approach based on the double aromatic nucleophilic substitution (SNACK) was developed. This methodology allows a facile incorporation of heterocyclic motifs into macrocyclic rings and rapid synthesis of a significant number of structurally diverse macrocycles. SNACK macrocyclisation enables preparation of stable diastereoisomers of conformationally restricted macrocycles (atropisomers). Practical application of SNACK macrocyclisation in a drug discovery project was exemplified by the identification of high affinity macrocyclic binders of B-cell lymphoma 6 (BCL6).


 Please wait while we load your content...
Please wait while we load your content...