Synthesis and photophysical evaluation of polarity sensitive push–pull isoquinolines and their alkynyl precursors†
Abstract
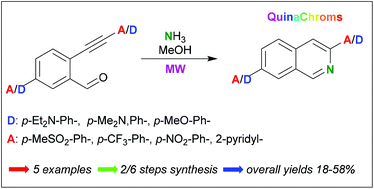
Two sets of unprecedented push–pull isoquinolines, characterized by an opposite “dipolar moment” with respect to the longitudinal axis of the molecule, have been prepared. The key step of the approach is the microwave-promoted domino imination/cycloisomerization of 2-alkynyl benzaldehydes in the presence of methanolic ammonia. Absorption spectra and emission spectra of the D-π-A isoquinolines and their alkynyl precursors in nine different solvents have been recorded. The absolute QYs of all compounds have been recorded in three solvents with different polarities, i.e. toluene, DMSO and ethanol. Among the D-π-A isoquinolines prepared – nicknamed QuinaChroms – two compounds characterized by opposite dipolar moments, i.e. 3-(4-methoxyphenyl)-7-nitroisoquinoline 1a and N,N-diethyl-3-(4-(methylsulfonyl)phenyl)isoquinolin-7-amine 2b displayed more interesting photophysical profiles, whereas 5-(diethylamino)-2-(A)arylethynylbenzaldehydes precursors 8a–c – having a free aldehyde group that is suitable for possible conjugation − exhibited strong fluorescence and wide Stokes shifts. These products are interesting for potential use as polarity-sensitive fluorescent probes or advanced functional materials.


 Please wait while we load your content...
Please wait while we load your content...