Photoredox halogenation of quinolones: the dual role of halo-fluorescein dyes†
Abstract
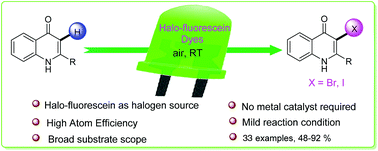
An efficient C-3 halogenation of quinolin-4-ones is reported with halogenated fluorescein dyes which serve both as a halogen source and photocatalyst. This reaction shows broad substrate scope and gives good to excellent yields of C-3 brominated/iodinated quinolones with eosin Y/rose bengal in green light under ambient conditions. The mechanistic investigations suggest a radical pathway involving the oxidative dehalogenation of the dye in the presence of air.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...