Recent advances in the functionalization of polyfluoro(aza)aromatics via C–C coupling strategies
Abstract
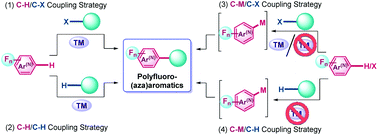
Polyfluoro(aza)aromatic compounds are of interest in various fields of practical applications, such as medicinal and agrochemistry, materials science and advanced technologies. The C–C coupling reactions are known to be a promising synthetic tool to create challenging fluorinated molecules of diverse architectures. In this review, we have summarized the recent advances in the functionalization of polyfluoro(aza)aromatics via both transition metal-catalyzed and metal-free C–C coupling reactions for the period from 2006 to the beginning of 2021. Also, mechanistic features for chemical transformations of fluoroarene scaffolds and new opportunities for practical applications of the designed fluorinated molecules have been highlighted.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...