Palladium-catalyzed aerobic oxyarylthiolation of alkynone O-methyloximes with arylhydrazines and elemental sulfur†
Abstract
A novel and practical palladium-catalyzed aerobic oxyarylthiolation of alkynone O-methyloximes for the assembly of 4-sulfenylisoxazole derivatives using S8 and arylhydrazines as the S-aryl sources is accomplished. In the presence of 0.1 mol% of IPr–Pd–allyl–Cl as the catalyst and O2 (1 atm) as the sole oxidant, both alkynone O-methyloximes and arylhydrazines are suitable substrates, delivering diverse 4-sulfenyl isoxazoles in moderate to good yields with good functional group tolerance. Notably, the phenyl diazonium salt and sodium phenyl sulfinate are also suitable arylation reagents, providing an alternative synthetic strategy to access structurally diverse 4-sulfenyl isoxazoles.
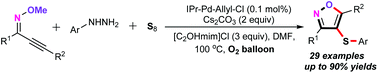
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...