Understanding the binding properties of phosphorylated glycoluril-derived molecular tweezers and selective nanomolar binding of natural polyamines in aqueous solution†
Abstract
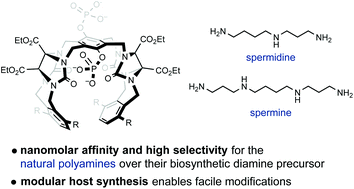
A modular synthetic platform for the construction of flexible glycoluril-derived molecular tweezers was developed. The binding properties of four exemplary supramolecular hosts obtained via this approach towards 16 organic amines were investigated by means of 1H NMR titration. In this work, we compare the Ka values obtained this way with those of three structurally related molecular tweezers and provide a computational approach towards an explanation of the observed behavior of those novel hosts. The results showcase that certain structural modifications lead to very potent and selective binders of natural polyamines, with observed binding of spermine below 10 nM.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...