Iodine-catalyzed thioallylation of indoles using Bunte salts prepared from Baylis–Hillman bromides†
Abstract
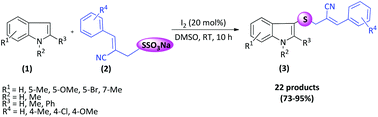
Metal-free iodine-catalyzed regioselective thioallylation of indoles has been accomplished at room temperature using Bunte salts prepared from Baylis–Hillman bromides. The resulting multi-functional C3 thioallylated indoles exhibit ample structural diversity and good functional group tolerance.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...