Ruthenium(ii) complexes with N-heterocyclic carbene–phosphine ligands for the N-alkylation of amines with alcohols†
Abstract
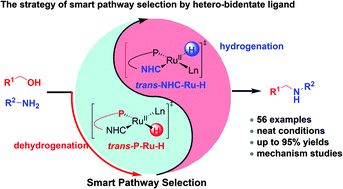
Metal hydride complexes are key intermediates for N-alkylation of amines with alcohols by the borrowing hydrogen/hydrogen autotransfer (BH/HA) strategy. Reactivity tuning of metal hydride complexes could adjust the dehydrogenation of alcohols and the hydrogenation of imines. Herein we report ruthenium(II) complexes with hetero-bidentate N-heterocyclic carbene (NHC)-phosphine ligands, which realize smart pathway selection in the N-alkylated reaction via reactivity tuning of [Ru–H] species by hetero-bidentate ligands. In particular, complex 6cb with a phenyl wingtip group and BArF− counter anion, is shown to be one of the most efficient pre-catalysts for this transformation (temperature is as low as 70 °C, neat conditions and catalyst loading is as low as 0.25 mol%). A large variety of (hetero)aromatic amines and primary alcohols were efficiently converted into mono-N-alkylated amines in good to excellent isolated yields. Notably, aliphatic amines, challenging methanol and diamines could also be transformed into the desired products. Detailed control experiments and density functional theory (DFT) calculations provide insights to understand the mechanism and the smart pathway selection via [Ru–H] species in this process.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...