Metal-free reduction of unsaturated carbonyls, quinones, and pyridinium salts with tetrahydroxydiboron/water†
Abstract
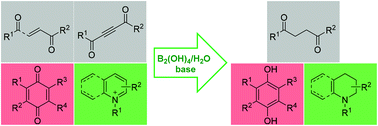
A series of unsaturated carbonyls, quinones, and pyridinium salts have been effectively reduced to the corresponding saturated carbonyls, dihydroxybenzenes, and hydropyridines in moderate to high yields with tetrahydroxydiboron/water as a mild, convenient, and metal-free reduction system. Deuterium-labeling experiments have revealed this protocol to be an exclusive transfer hydrogenation process from water.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...