Palladium-catalyzed carbonylative cyclization of benzyl chlorides with anthranils for the synthesis of 3-arylquinolin-2(1H)-ones†
Abstract
An efficient carbonylative procedure for the synthesis of 3-arylquinoin-2(1H)-ones has been established. Through a palladium-catalyzed aminocarbonylation of benzyl chlorides with anthranils, a variety of 3-arylquinoin-2(1H)-one products were obtained in moderate to excellent yields with good functional group tolerance.
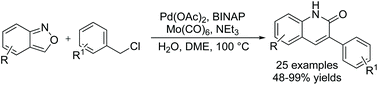
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...