Direct and straightforward transfer of C1 functionalized synthons to phosphorous electrophiles for accessing gem-P-containing methanes†
Abstract
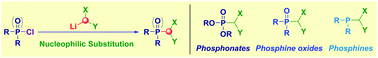
The direct transfer of different α-substituted methyllithium reagents to chlorinated phosphorous electrophiles of diverse oxidation state (phosphates, phosphine oxides and phosphines) is proposed as an effective strategy to synthesize geminal P-containing methanes. The methodology relies on the efficient nucleophilic substitution conducted on the P-chlorine linkage. Uniformly high yields are observed regardless the specific nature of the carbanion employed: once established the conditions for generating the competent nucleophile (LiCH2Hal, LiCHHal2, LiCH2CN, LiCH2SeR etc.) the homologated compounds are obtained via a single operation. Some P-containing formal carbanions have been evaluated in transferring processes, including the carbonyl-difluoromethylation of the opioid agent Hydrocodone.


 Please wait while we load your content...
Please wait while we load your content...